Calculate the standard enthalpy of formation Key Takeaways Key Points The standard state of a material is a reference point for the materials thermodynamic state properties. A yields B DGdelta Go 128 kJ C yields 2B DGo 455 kJ A yields C DGo -182 kJ Report your answer to three significant figures.
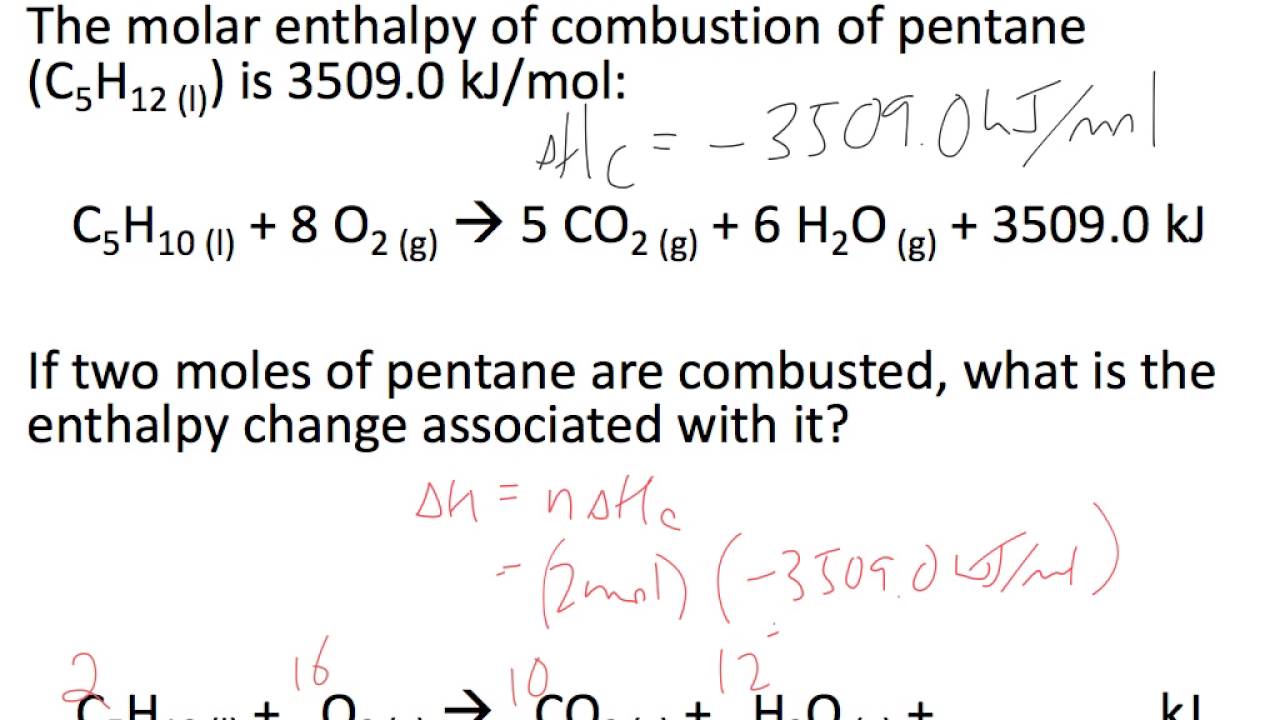
Molar Enthalpy Calculations Youtube
Use the following information to calculate the standard Gibbs free energy for the reaction.
. C2H5OHℓ 72O2g --- 2CO2g 3H2Oℓ Before launching into the solution notice I used standard enthalpy of combustion This is a very common chemical reaction to take something. Moles mass molar mass Calculate energy required to change temperature of water. Simply plug your values into the formula H m x s x T and multiply to solve.
To calculate the enthalpy of solution heat of solution using experimental data. Q m C g ΔT. So we convert the carefully measured mass in to moles by dividing by molar mass.
The standard heat of reaction is -113 kJ. Molar Heat Capacity Heat Number of MolesChange in Temperature cm Q nT This formula uses 3 Variables Variables Used Heat - Heat is the form of energy that is transferred between systems or objects with different temperatures flowing from the high-temperature system to the low-temperature system. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators.
Nov 18 2019 Click here to get an answer to your question Compute the molar enthalpy of combustion of glucose C6 H12O6. 303 calculate the heat energy change from a measured temperature change using the expression Q mcΔT. Calculate moles of solute.
Calculate moles n of fuel used. In molar heat of neutralization problems n CV where C concentration in M molesL. As the acid and the base are fully dissociated and neither the cation B nor the anion A are involved in the neutralization reaction.
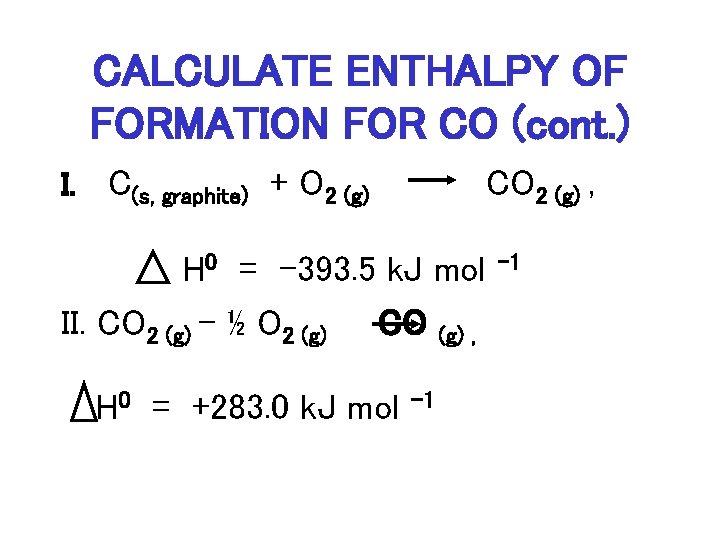
Molar enthalpy D H n. ΔΗ combustion KJ Substance ΔΗ kJmole -2777 00 CHOH1 02g CO2g H2Og -3935 -2418. Calculate the molar heat of combustion.
305 Triple only draw and explain energy level diagrams. For volume use meter cubed or m3 as the standard unit of measurement. List the known quantities and plan the problem.
The balanced equation is. Under the law of conservation of energy the shift of internal energy is proportional to the heat transmitted to the device minus the work performed by it. Amount of energy released or absorbed is calculated.
Calculate the standard molar enthalpy for the complete combustion of liquid ethanol C2H5OH using the standard enthalpies of formation of the reactants and products. In the symbols the enthalpy H is equivalent to the sum of the internal energy E and the strain P and volume V of the system. As previously mentioned enthalpy has the following equation.
24 104g 1 mole NaOH 39997g 600 106 moles NaOH You know that the enthalpy of dissolution when 600 106 moles of sodium hydroxide are dissolved in water so use this info to find the enthalpy of dissolution when 1 mole of the salt dissolves 1mole NaOH 6322 J 600 106moles NaOH 1054 107 J Finally convert this to kilojoules. Once you have m the mass of your reactants s the specific heat of your product and T the temperature change from your reaction you are prepared to find the enthalpy of reaction. C6 H12O6 s 6 O2.
The enthalpy change for this reaction is -5762 kJmol at 25 C. First write the balanced equation for the reaction. N m M.
The molar heat capacity of solid aluminium is 244 J K 1 mol1 at 25C. H E PV respectively. Q amount of energy released or absorbed.
H U PV For pressure use pascal as the standard unit of measurement. Up to 24 cash back How to calculate standard molar enthalpy change example problem. -5762 kJmolFrom this the standard enthalpy change H is obtained by division with the amount of substance in moles involved.
What is the change in. Calculate the standard enthalpy of combustion for the following reaction. 1 If the solute and the solvent are in their standard states you can also write ΔH osol Refer to Standard Enthalpy heat of Formation and Reaction 2 You may also units of cal mol -1 or kcal mol -1 1 calorie 418 joules 1 cal 418 J For conversions.
90 grams of charcoal C were completely consumed in a bomb calorimeter. Applying the equation form the text. H H products H reactants Definition of Enthalpy The precise definition of enthalpy H is the sum of the internal energy U plus the product of pressure P and volume V.
Then apply the equation to calculate the standard heat of reaction for the standard heats of formation. 304 calculate the molar enthalpy change ΔH from the heat energy change Q. Up to 24 cash back How to calculate molar enthalpy of formation The standard enthalpy of formation refers to the enthalpy change when one mole of a compound is formed from its elements.
Use the formula H m x s x T to solve. You can calculate changes in enthalpy using the simple formula. N number of moles of reactant.
How do you define enthalpy. Amount of energy heat released or absorbed per mole of solute is. V volume in litres.
But you can also use kilogram per meter per second-squared or kg m_s2.

Enthalpies Of Formation Chemsitry Tutorial Youtube
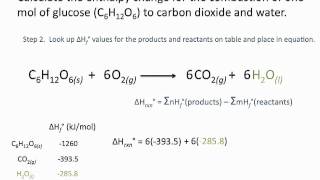
0 Comments